Summary:
• Scientists have developed a new thin film that helps produce hydrogen from water using sunlight more efficiently.
• This film assembles itself and is easier to make than traditional materials used in solar water splitting.
• The technology could make solar-powered hydrogen production more practical and affordable
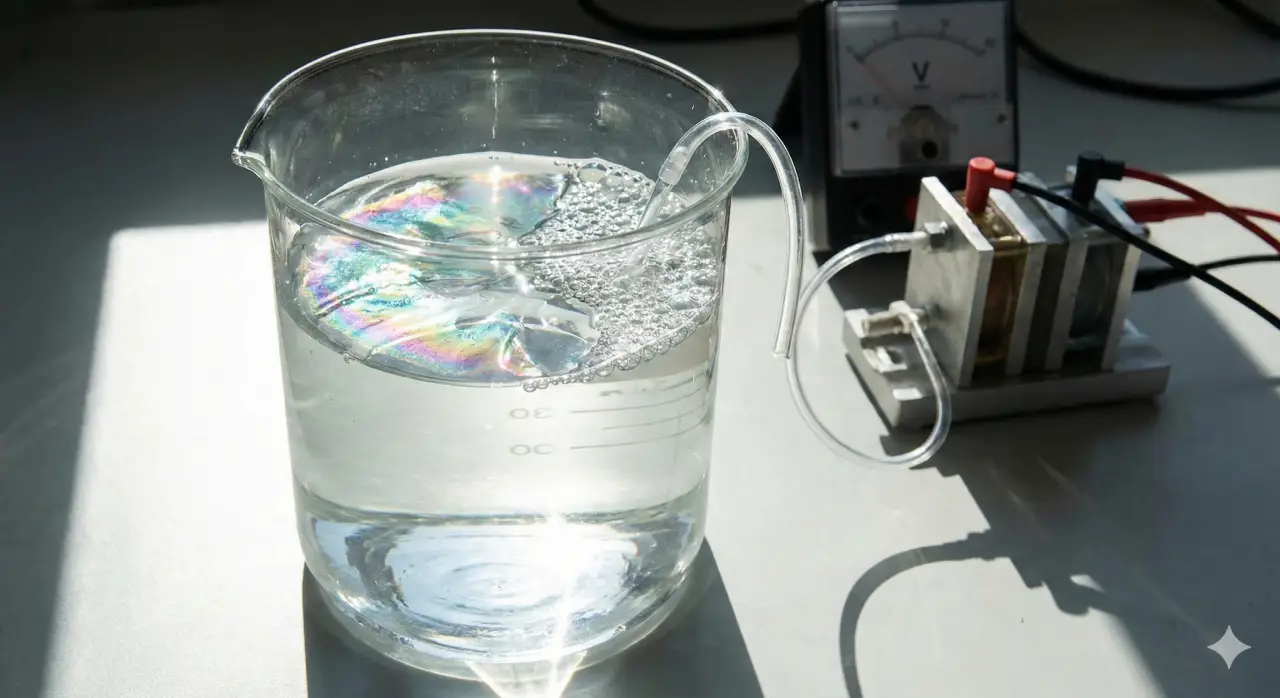
Hydrogen is often called a clean fuel for the future, especially if it can be made using renewable energy. One promising way to produce hydrogen is by using sunlight to split water into hydrogen and oxygen. This process, known as solar water splitting, could provide a sustainable source of hydrogen fuel. However, making this process efficient and affordable remains a challenge.
A research team led by Professor Han-Hee Cho at UNIST has introduced a new type of material that could help solve this problem. The team developed a special thin film, called a self-assembled monolayer (SAM), made from molecules based on naphthalimide. This film is designed to help electrons move more easily during the water-splitting process.
Here’s how the process works: When sunlight hits a photoelectrode (a special light-sensitive material) submerged in water, it creates excited electrons. These electrons need to move quickly and efficiently through the material to drive the chemical reaction that splits water into hydrogen and oxygen.
Traditionally, a layer of metal oxide is used to help transfer electrons in this setup. However, metal oxide layers are often thick and not very efficient at moving electrons. They can also be expensive and energy-intensive to produce.
The new molecular film developed by Professor Cho’s team acts as a much thinner and more efficient bridge for electrons. Because these molecules can assemble themselves into a single layer, the manufacturing process is simpler and uses less energy.
A key feature of the new film is its “push-pull” design. Each molecule contains parts that donate electrons and parts that attract them. This arrangement creates a strong internal electric field, making it easier for electrons to move through the layer. This effect, called quantum tunneling, means electrons can pass through the barrier more easily, improving the overall efficiency of the device.
In laboratory tests, photoelectrodes coated with this new film achieved a higher current density than previous organic-based devices. Higher current density means more electrons are moving, which leads to faster and more efficient hydrogen production.
This breakthrough could help make solar-powered hydrogen production more practical and affordable. Because the film is based on organic molecules and can be produced over large areas, it could be scaled up for industrial use. Beyond hydrogen production, the same push-pull molecular design could also improve other devices that rely on efficient electron movement, such as solar cells, LEDs, and sensors.
This research highlights an exciting step forward in the search for sustainable materials that support clean energy technologies.
The findings were published in ACS Energy Letters (2025) by researchers Euimin Lee, Donghyun Lee, Gyujin Jang, and others, led by Professor Han-Hee Cho from the Department of Materials Science and Engineering at UNIST. The project received support from the Korean National Research Foundation (NRF), the InnoCORE program of the Ministry of Science and ICT (MSIT), and the Swiss ETH Leading House Asia.