Image for Illustrative Purposes Only.
Summary :
• Most current batteries use rare materials and flammable liquids that can be risky and expensive.
• Researchers are developing solid-state batteries using sodium, which is more common and safer.
• New materials could lead to batteries that last longer, cost less, and work well in many conditions.
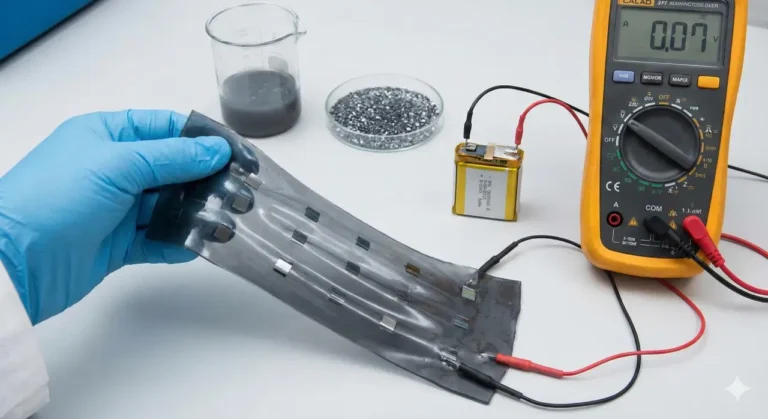
Batteries power much of our daily lives, from smartphones to electric cars. But the batteries we use today have some big drawbacks. They often contain flammable liquids that can catch fire and rely on rare elements like lithium, which are expensive and sometimes difficult to source.
A team of researchers, led by Professor Yang Zhao, is working on a new kind of battery that could solve many of these problems. Instead of lithium, they are focusing on sodium—a much more common and affordable element. Sodium is found everywhere, including in table salt, making it a promising alternative for large-scale battery production.
One of the biggest changes in their approach is the use of a solid-state electrolyte. In traditional batteries, the electrolyte is a liquid that helps ions (charged particles) move between the battery’s positive and negative ends. However, these liquids are flammable and can be dangerous if the battery is damaged. Solid-state electrolytes replace the liquid with a solid material, making the battery much safer and potentially allowing it to store more energy.
The challenge has been to get sodium ions to move easily through a solid. Zhao and his team developed a new material that combines sulfur and chlorine, which helps sodium ions travel quickly and reliably. The sulfur makes it easier for the ions to move, while also making the material stronger overall.
This new material is not only good at conducting sodium ions but is also stable under heat and pressure. That means batteries made with it could last through many cycles of charging and discharging and work well in hot or cold environments. Unlike some other solid-state designs, this material does not break down when it touches other parts of the battery.
To understand exactly how their new material works, the researchers used powerful X-ray tools at the Canadian Light Source. These tools let them see how the ions move and how the material holds together—details that are hard to spot with regular lab equipment.
While these new sodium solid-state batteries are not yet ready for everyday use, the research is moving quickly. If successful, these batteries could be safer, cheaper, and longer-lasting than what we have today, making them a promising solution for a cleaner and more reliable energy future.
This research was published in the journals Advanced Functional Materials and Advanced Materials by Professor Yang Zhao and colleagues at Western University.