Image for Illustrative Purposes Only.
Summary:
• Solid-state batteries can be safer and store more energy than traditional types.
• Organic materials like indigo dye may help these batteries work better and more sustainably.
• New research shows careful mixing of indigo and battery parts can increase performance, even in cold conditions.
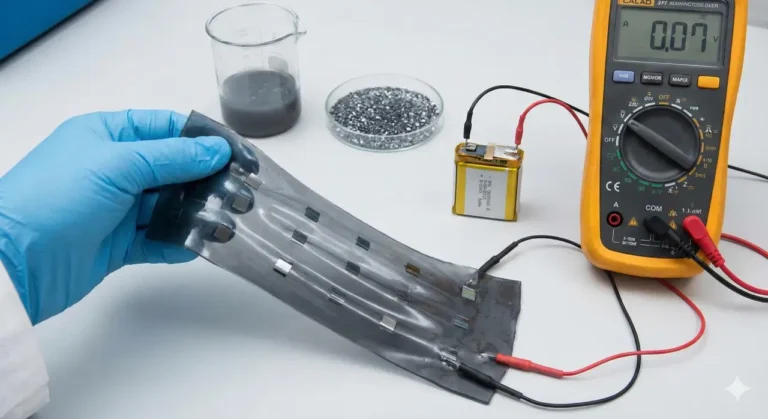
Batteries are everywhere in our daily lives, from phones to electric cars. As we look for safer and more environmentally friendly options, solid-state batteries have gained attention. Unlike regular batteries that use liquid to move lithium ions, solid-state batteries use solid materials. This change can make batteries safer and able to hold more energy. However, making these batteries work well with green, organic ingredients has been a challenge.
Organic materials are attractive because they are often less expensive and better for the environment. But when these materials are mixed with the solid parts of a battery, they usually react in ways that make the battery unstable. Most scientists have tried to stop these reactions to keep the battery working properly.
A new study, however, has taken a different approach. Researchers explored what happens if you carefully control the reaction between an organic material called indigo—a natural blue dye—and the solid electrolyte inside the battery. Instead of causing problems, this managed reaction actually made the battery work better.
Here’s how it works: Indigo can store and release lithium, which is needed for the battery to provide power. At the same time, indigo helps the solid electrolyte store energy too. This teamwork between the dye and the electrolyte means the battery can hold much more energy than if either material worked alone.
Another exciting result is that the battery performed well not only at room temperature but also in cold conditions, down to minus ten degrees Celsius. This is unusual for batteries that use organic materials, as they often struggle in the cold.
The researchers say this is one of the best performances ever reported for this type of battery. It shows that natural molecules like indigo could help solve long-standing problems and make solid-state batteries more practical and sustainable.
There is still work to do before these batteries are ready for everyday use. The next steps will focus on making the reaction even more stable and allowing the battery to store more energy in a small space. If successful, this could bring us closer to greener, safer batteries for everything from portable devices to electric vehicles.
This research was conducted by Qihang Yu (first author), Li, and their team at Concordia’s Gina Cody School of Engineering and Computer Science.